Many adults with ADHD and depression wonder whether it’s safe to combine their medications.
Taking Zoloft and Vyvanse together is common in clinical practice, but the combination requires careful monitoring because both drugs affect brain chemistry in ways that can interact.
This article explains the risks, benefits, and practical steps clinicians use to make the combination safer.
Understanding How Zoloft and Vyvanse Work
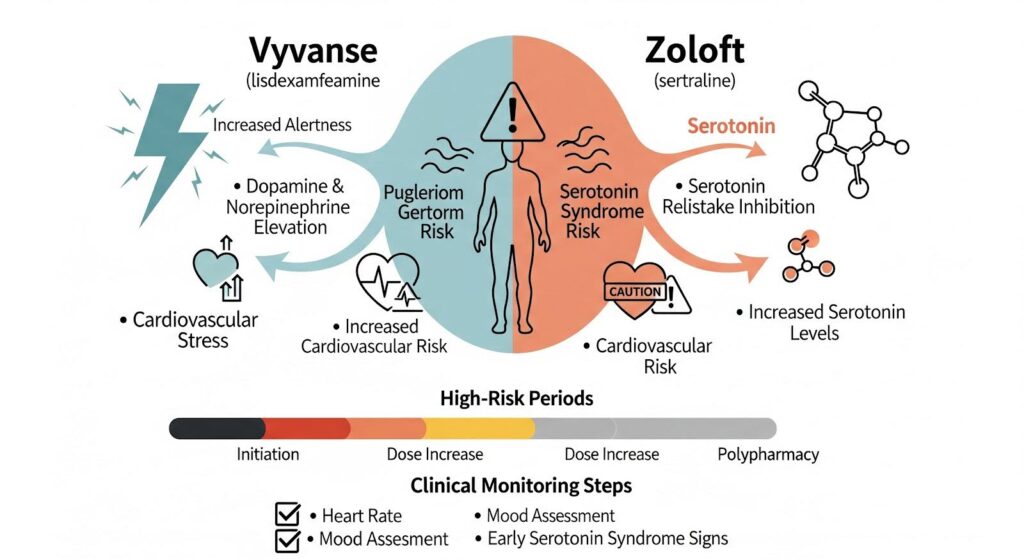
Sertraline, sold as Zoloft, is a selective serotonin reuptake inhibitor that treats depression and anxiety by increasing serotonin levels in the brain.
Lisdexamfetamine, marketed as Vyvanse, is a prodrug that converts to dextroamphetamine and helps ADHD by boosting dopamine and norepinephrine.
At higher doses, amphetamines can also increase serotonin release, which creates a potential overlap with SSRIs.
The FDA label for Vyvanse explicitly warns about serotonin syndrome when the drug is combined with serotonergic agents like SSRIs.
Serotonin syndrome is a rare but serious condition marked by agitation, confusion, rapid heart rate, high blood pressure, tremor, muscle rigidity, and fever.
The risk is highest during the first week after starting either medication or increasing the dose.
Sertraline also moderately inhibits an enzyme called CYP2D6, which helps metabolize amphetamines.
This means Zoloft can slightly increase Vyvanse exposure in the body, though the effect is smaller than with stronger inhibitors like fluoxetine or paroxetine.
Because of this interaction, the Vyvanse label advises starting at a lower dose and monitoring closely when serotonergic drugs or CYP2D6 inhibitors are involved.
Can You Take Vyvanse and Zoloft Together Safely?
Yes, but only under medical supervision with a structured plan. The NICE guideline on ADHD recommends baseline cardiovascular screening before starting stimulants, including a full medical history, blood pressure and heart rate measurement, and assessment for cardiac risk factors.
An ECG is not routinely required unless specific risk features are present, such as a family history of sudden death under age 40, exertional syncope, chest pain, or a heart murmur.
When doctors prescribe Zoloft and Vyvanse together, they typically follow a conservative titration protocol. If you’re already on sertraline, your clinician will likely start lisdexamfetamine at a lower than usual dose and increase it slowly every one to two weeks based on how you respond and tolerate the medication.
If you’re already on Vyvanse, sertraline is usually started at a standard or low dose and increased gradually.
The key principle is to avoid rapid changes in both medications at the same time, which makes it easier to identify the source of any side effects.
Patient education is critical. You should learn the early signs of serotonin syndrome, which include agitation, restlessness, tremor, sweating, diarrhea, and muscle twitching.
More serious symptoms include muscle rigidity, high fever, confusion, and seizures. If you notice a cluster of these symptoms, especially within the first week after a dose change, seek medical care immediately.
Vyvanse and Zoloft Interactions: What the Research Shows
A 2021 review of serotonin syndrome in children and adolescents found that symptoms typically appear within 24 hours of a dose increase, overdose, or addition of another serotonergic drug.
While the review focused on younger patients, the underlying pharmacology applies to adults. One case involved a teenager on sertraline 150 mg who developed serotonin syndrome two days after starting methylphenidate, a stimulant with weaker serotonergic effects than amphetamines.
This underscores that even therapeutic doses can precipitate toxicity when serotonergic tone is elevated.
The PDR drug interaction monograph for amphetamines lists SSRIs and SNRIs as moderate-risk combinations, with the highest risk during initiation and dose escalations.
The monograph advises clinical vigilance and patient counseling on early warning signs.
From a cardiovascular standpoint, recent data suggest small but measurable risks with long term stimulant use. An ACC press release in 2024 reported that stimulant prescriptions were associated with an increased likelihood of developing cardiomyopathy in young adults, with an estimated absolute risk of about 1 in 2,000 per year or roughly 1 in 500 over 10 years.
A JACC journal scan of a nationwide cohort found elevated 10 year risk of stroke and heart failure with increasing dosage and prior use, though no association with acute coronary syndrome.
These observational studies cannot prove causation, but the dose response patterns support prudent dose minimization and monitoring.
A 2022 meta analysis of nearly 3.9 million subjects found no statistically significant association between ADHD medications and cardiovascular disease overall, including in those with pre existing CVD.
However, heterogeneity was high, meaning the absence of a pooled effect does not rule out risk in subgroups.
Common Side Effects When Taking Zoloft and Vyvanse
The most frequently reported side effects during combined use are insomnia, decreased appetite with weight loss, anxiety or nervousness, headache, and nausea.
Both drugs independently cause sleep disturbance, so the combination often amplifies this effect. The Vyvanse label recommends morning dosing to reduce insomnia.
If sertraline is activating for you, taking it in the morning may help; if it’s sedating, evening dosing might be better.
Appetite suppression and weight loss are hallmark stimulant effects. When combined with Zoloft, the appetite impact is driven primarily by Vyvanse.
Clinicians typically track weight monthly, especially in younger adults, and may suggest eating a substantial breakfast before dosing or adding nutrient dense snacks later in the day when appetite returns.
Anxiety, irritability, and emotional activation are common during the first few weeks, particularly during dose adjustments.
A FAERS analysis of pediatric and adolescent reports found disproportionate signals for drug induced mental disorders with both sertraline and lisdexamfetamine, including anxiety and irritability. Slower titration, baseline anxiety screening, and behavioral support can help manage these symptoms.
Headache is common with both agents and usually responds to hydration and simple analgesics.
Nausea and gastrointestinal upset reflect sertraline’s SSRI profile and often improve when the medication is taken with food or titrated more slowly.
Mild increases in heart rate and blood pressure are expected with stimulants.
NICE recommends reducing the dose and referring to a specialist if sustained resting heart rate exceeds 120 beats per minute, arrhythmia develops, or blood pressure rises above the 95th percentile on two separate occasions.
Monitoring Checklist for Combined Use
- Baseline: Blood pressure, heart rate, weight, cardiac history, medication review, sleep and appetite assessment
- Week 1–2: Vitals, activation symptoms, sleep quality, gastrointestinal symptoms, mood and anxiety, ADHD rating scales
- Week 3–6: Repeat assessments; adjust one medication at a time based on benefits and risks
- Month 2–3: Stabilize doses; monitor function at work or school, appetite, and weight
- Quarterly: Vitals, adherence, life changes; maintain or adjust regimen as needed
When Doctors Avoid the Combination?
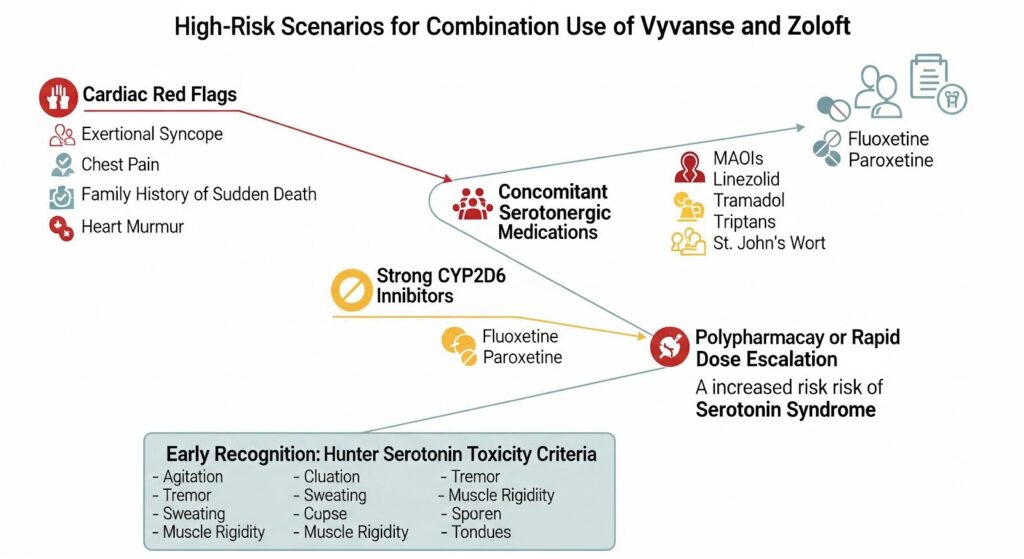
Certain situations make the combination riskier or inappropriate. If you have cardiac red flags such as exertional syncope, unexplained chest pain, a family history of sudden death under age 40, or a heart murmur, your doctor should refer you to cardiology before starting any stimulant.
If you’re taking MAOIs or the antibiotic linezolid, which have strong serotonergic effects, the risk of serotonin syndrome is substantial and the combination should be avoided.
If you’re on a strong CYP2D6 inhibitor like fluoxetine or paroxetine, your doctor may switch you to sertraline or escitalopram before adding Vyvanse, or proceed with a very low stimulant dose and close monitoring.
Concomitant use of other serotonergic agents such as tramadol, triptans, or St. John’s Wort increases risk and should be minimized or avoided.
A review of serotonin syndrome emphasizes that the syndrome is a spectrum of toxicity most often precipitated by polypharmacy and dose increases.
Early recognition using diagnostic criteria like the Hunter Serotonin Toxicity Criteria improves outcomes and helps differentiate serotonin syndrome from other conditions.
Practical Steps to Minimize Risk
Start with a full medication reconciliation to identify all serotonergic drugs and CYP2D6 inhibitors. Measure baseline vitals and screen for cardiac risk factors.
If you have any red flag features, get cardiology clearance before starting a stimulant.
Educate yourself on serotonin syndrome early signs: agitation, tremor, sweating, diarrhea, muscle twitching. Know to stop both medications and seek urgent care if a cluster of symptoms develops.
Avoid over the counter serotonergic products like dextromethorphan cough syrups and supplements like St. John’s Wort.
Track your blood pressure and heart rate at home if possible during the first few weeks. Report persistent tachycardia or blood pressure elevation to your clinician.
Schedule weekly to biweekly check ins during titration, then every one to three months once stable.
If you experience persistent insomnia, discuss dose timing with your doctor. Morning Vyvanse and adjusting sertraline timing based on whether it activates or sedates you can help.
If anxiety or irritability becomes impaired, consider slowing the titration or adding cognitive behavioral strategies.
The Role of Genetics in Drug Interactions
About 5 to 10 percent of people of European descent are poor metabolizers of CYP2D6, meaning they break down certain drugs more slowly. A pharmacogenetics review notes that for amphetamine like agents, CYP2D6 accounts for a smaller fraction of overall clearance than expected, so genetic variability has less impact on acute toxicity than with classic CYP2D6 substrates like atomoxetine. Routine genetic testing is not mandatory before combining Zoloft and Vyvanse, but it may be considered if you have unexpected sensitivity or adverse effects.
A 1997 pharmacokinetic study compared CYP2D6 inhibition among SSRIs and found that paroxetine and fluoxetine are strong inhibitors, sertraline is moderate, and citalopram and escitalopram are weak. This hierarchy helps clinicians choose the SSRI least likely to increase amphetamine exposure when a stimulant is needed.
Why This Combination Matters
For many adults, co occurring ADHD and depression or anxiety significantly impair function at work, school, and in relationships. Treating one condition while leaving the other untreated often leads to suboptimal outcomes. The CADDRA practice guidelines emphasize individualized, multimodal treatment plans that incorporate both pharmacological and psychosocial interventions.
When executed with careful baseline screening, conservative titration, patient education, and regular monitoring, the combination of sertraline and lisdexamfetamine can be both safe and effective. The absolute risk of serious adverse events like serotonin syndrome or cardiomyopathy appears low in appropriately selected patients, but vigilance during the first weeks and adherence to monitoring thresholds are essential safeguards.
If you’re struggling with ADHD and co occurring mood or anxiety symptoms, you don’t have to navigate treatment alone. At MARR, our team integrates evidence based therapies like CBT and DBT with a supportive therapeutic community to address the full spectrum of your needs. Reach out to our team to learn how we can help you build a personalized recovery plan.